Mitophagy: The process that keeps our cells healthy
Mitophagy is the version of autophagy that applies to mitochondria. This potent quality control system clears away damaged mitochondria.
Our cells constantly clean up the cellular garbage and recycle their components.[1] This process is called autophagy and is so important for our cells that the 2016 Nobel Prize in Physiology and Medicine went to Yoshinori Ohsumi for his discoveries related to autophagy mechanisms. Mitophagy is the version of autophagy that applies to mitochondria. The science around this is relatively new and it’s attracting a lot of interest from world-class scientists.
Panta rhei (πάντα ῥεῖ), “everything flows” said the Greek philosopher Heraclitus. This flow is happening not only in our visible traits and behaviors but also inside each of our “invisible” cells. In fact, our cells constantly build new molecular components and then remove and recycle them when they get damaged or exhausted after use. As a consequence, our cells are kept younger than the body as a whole. A team of Swedish researchers found that the average cell in the body is less than 10 years old.[2]
Autophagy is the biological process taking care of this cellular cleanup. Mitochondria are our cellular powerhouses and are so essential that we have a dedicated form of autophagy that applies to them. It is called mitophagy.[3] Mitophagy promotes the natural flow of our mitochondria, removing the dysfunctional organelles and priming the biogenesis of healthy new ones.
What exactly is mitophagy?
The word mitophagy comes from Greek, and literally means mitochondrial digestion (from phagos). This process is critical to keeping our mitochondria healthy. When mitophagy is blocked, dysfunctional mitochondria start accumulating in the cells, a phenomenon witnessed during the aging process. This causes problems in our cellular health, with negative effects on the whole organism.[4]
How mitophagy works
Several biological processes stimulate our cells to recycle the less functional mitochondria. This includes exercise and calorie restriction. To start the process of mitophagy, mitochondria change the properties of specific proteins on their surface. These proteins, also known as receptors, function as a “flag”, allowing the cells to recognize and remove the “bad” mitochondria.
The most relevant receptor is called PINK1. To promote mitophagy, mitochondria exhibit a modified form of PINK1 that, in turn, recruits another protein called Parkin. When the PINK1-Parkin complex forms, cells initiate the process of mitochondrial elimination.[5]
To achieve this, an envelope is built around the bad mitochondria, and they are directed towards a structure called a lysosome. Continuing with our Greek lesson, lysosome means dissolving (lyso) bodies (soma). Indeed, mitochondria get engulfed by lysosomes and are “eaten” or digested by lysosomal enzymes and a low pH.[6]
At the end of the process, the unhealthy mitochondria are not harmful anymore – even better, their building blocks can be recycled for other purposes. The PINK1/Parkin axis is just one example of mitophagy regulation. Cells have developed many more such mechanisms, depending on the type of mitophagy inducer and tissue.
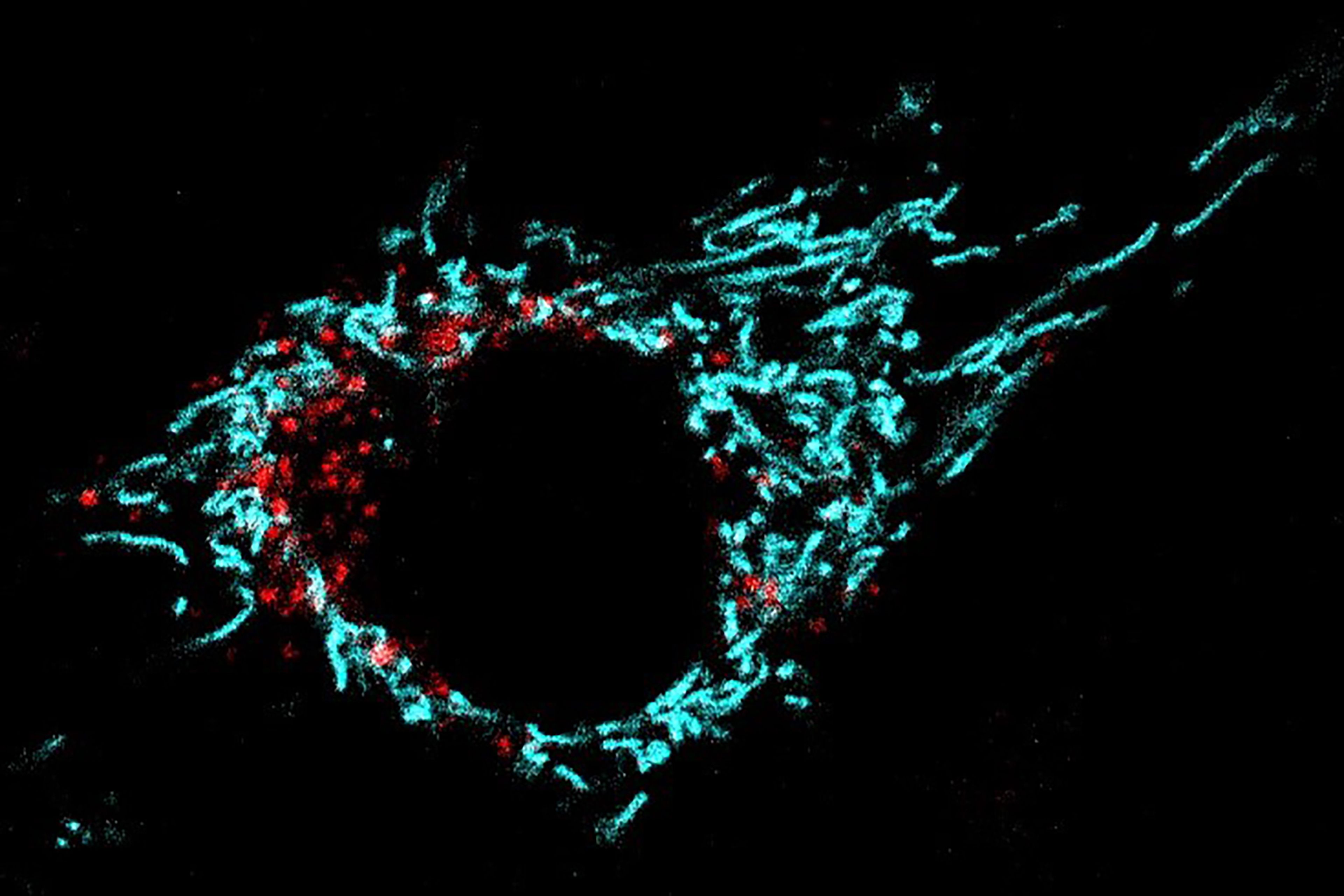
A muscle cell (C2C12 myoblast) stained with the mitochondrial marker mKEIMA. In cyan is represented the normal mitochondrial network. The red dots show mitochondria undergoing mitophagy. (Credit: Davide D’Amico/EPFL)
Mitophagy goes hand in hand with biogenesis
In the cellular flow, mitophagy cannot act alone – otherwise, our mitochondrial pool would be quickly drained. Indeed, when cells trigger mitophagy, they also follow instructions to build new mitochondria. This is called mitochondrial biogenesis. The coordination of biogenesis and mitophagy is the key to maintain the correct mitochondrial balance, also known as mitohormesis.[7]
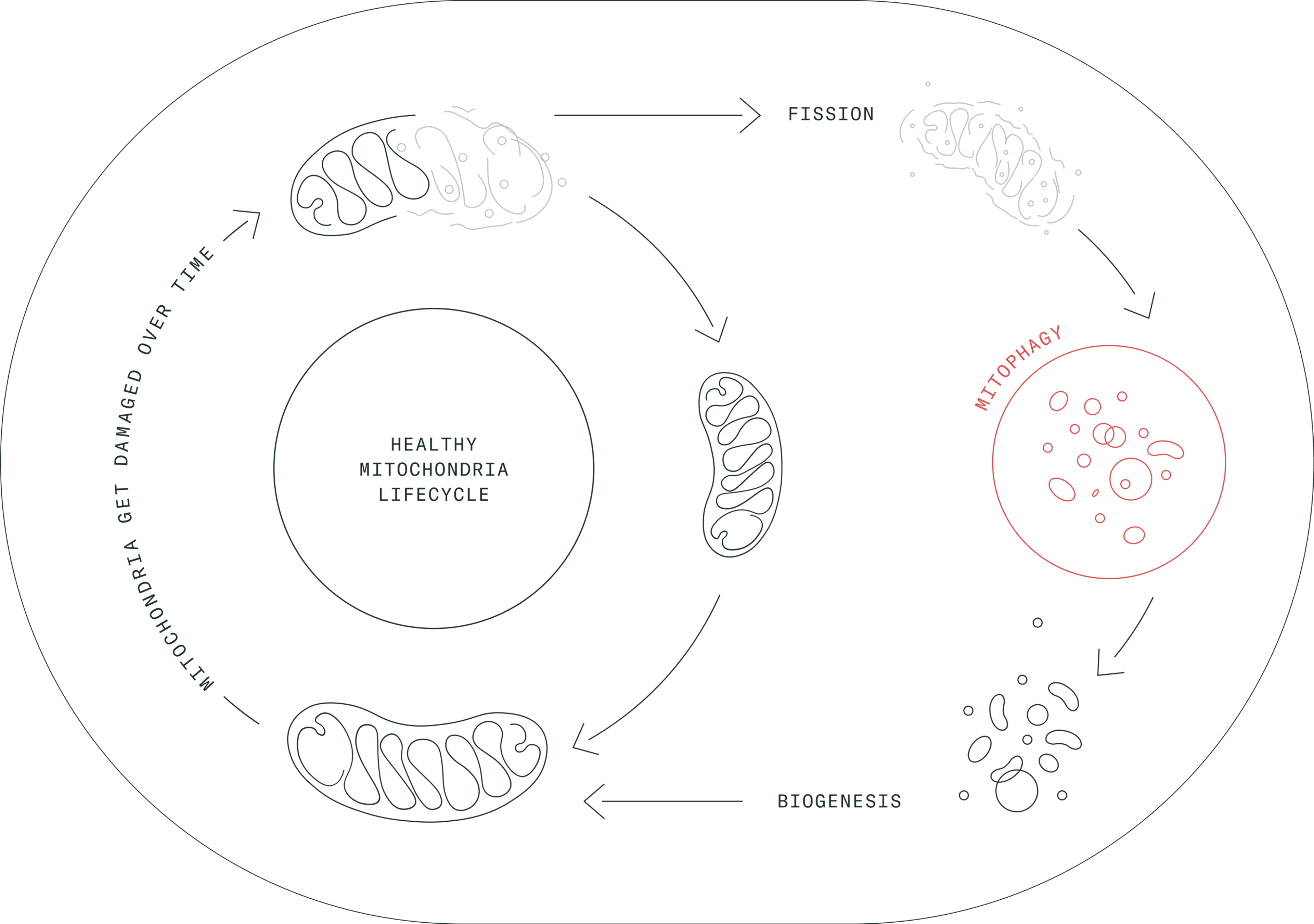
Mitophagy and biogenesis are key processes that are coordinated to maintain our cells healthy. (Image credit: Amazentis)
Mitophagy in aging and diseases
Mitochondrial dysfunction is a hallmark of aging and mitophagy plays a key role in this context. Several studies show that the rates of mitophagy decline with aging and this has been linked to several age-related disorders such as Parkinson’s and Alzheimer’s disease[8], as well as metabolic disease and diabetes.[9]
Recent studies have shown that Urolithin A is able to enhance cellular and mitochondrial health. Urolithin A is the first natural bioactive shown to improve mitochondrial function by activating mitophagy that has been rigorously tested in humans. Other compounds contribute to mitochondrial function, but Urolithin A is unique in promoting mitochondrial health via a combination of mitophagy and mitochondrial biogenesis.
As a result of an oral intervention with Urolithin A, nematode worms live longer, and rodents show increased endurance.[10] Urolithin A impacts not only muscle but also other tissues. Two very recent papers, published by independent groups, provide evidence that Urolithin A reduces inflammation and amyloid-beta aggregation, thereby improving cognitive function in mouse models of Alzheimer's disease[11]. Another study by scientists at the University of Louisville published data describing how Urolithin A treatment can enhance the gut barrier integrity in a mouse model for inflammatory bowel disease (IBD).[12]
Long-term human trials are underway, but we already know that in an interventional double-blinded, randomized, and placebo-controlled study, Mitopure (a proprietary and highly pure Urolithin A) is safe in humans and improves cellular and mitochondrial health in a comparable manner to what was found in worms and rodents.[13]
Our deeper understanding of mitochondrial biology points to mitophagy as a key mechanism for proper cellular function. Learning how to stimulate mitophagy in circumstances when it would otherwise decline may well be the way forward, as a way to keep our cells healthy – a pre-requisite for a healthier life.
Authors
Written by
Editorial Staff
References
- ↑
Dance, A., May 15, for the N. I. of H. | & ET, 2013 11:11am. Cellular Garbage Disposals Clean Up.Live Science Available at: https://www.livescience.com/31966-cells-garbage-disposal-crucial-processes-nigms.html. (Accessed: 25th February 2019).
- ↑
Spalding KL et al., Retrospective Birth Dating of Cells in Humans. 122, 1, July 15 , 2005.
- ↑
Palikaras, K., Lionaki, E. & Tavernarakis, N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol. 20, 1013–1022 (2018).
- ↑
Palikaras, K., Lionaki, E. & Tavernarakis, N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol. 20, 1013–1022 (2018).
- ↑
Bingol, B. & Sheng, M. Mechanisms of mitophagy: PINK1, Parkin, USP30 and beyond. Free Radic. Biol. Med. 100, 210–222 (2016).
- ↑
The Discovery of Lysosomes and Autophagy. Nature Education 3(9):49.
- ↑
Ploumi, C., Daskalaki, I. & Tavernarakis, N. Mitochondrial biogenesis and clearance: a balancing act. FEBS J. 284, 183–195 (2017).
- ↑
1. Palikaras, K., Lionaki, E. & Tavernarakis, N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol. 20, 1013–1022 (2018).
2. Sun, N., Youle, R. J. & Finkel, T. The Mitochondrial Basis of Aging. Mol. Cell 61, 654–666 (2016).
3. Fang, E. F. et al.Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat. Neurosci. 22, 401–412 (2019).
- ↑
Springer, M. Z. & Macleod, K. F. Mitophagy: Mechanisms and Role in Human Disease. J. Pathol. 240, 253–255 (2016).
- ↑
Ryu, D. et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat. Med. 22, 879 (2016).
- ↑
1. Fang, E. F. et al.Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat. Neurosci. 22, 401–412 (2019).
2. Gong Z et al., Urolithin A attenuates memory impairment and neuroinflammation in APP/PS1 mice. J Neuroinflammation. 2019 Mar 14;16(1):62
- ↑
Singh R et al., Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nature Communications. 10, Article number: 89 (2019)
- ↑
Singh, A. et al. Orally administered urolithin a is safe and modulates muscle and mitochondrial biomarkers in elderly. Innov. Aging. 1, 1223–1224 (2017)

·
Nutrition·
Studies·









